> 资讯
Magstim Passes Compliance with Strict European Union Medical Device Regulations
Expanding Magstim Access to Entire EU Marketplace
WHITLAND, United Kingdom, May 16, 2025 (GLOBE NEWSWIRE) -- Magstim has been granted EU MDR Certification for all transcranial magnetic stimulation (TMS) systems, demonstrating compliance with strict European Union Medical Device Regulations. This provides a path for Magstim TMS technology to be sold throughout the EU marketplace, and proves that the device is safe, clinically effective and has passed all requirements for testing and traceability.
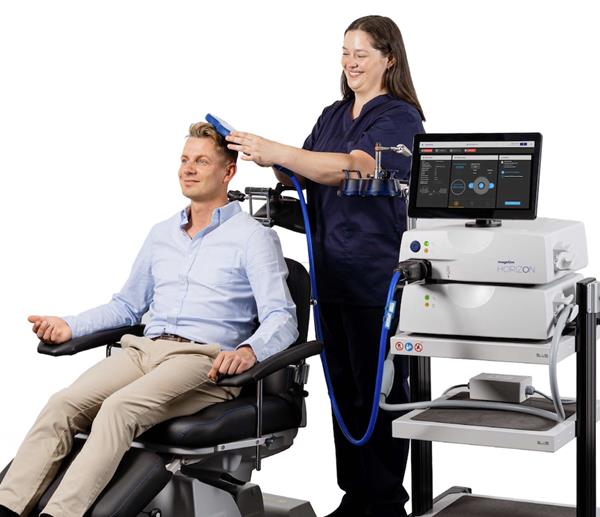
“The EU MDR is the most rigorous certification backed by verified scientific knowledge,” said Ronnie Stolec-Campo, CEO, Welcony. “As pioneers of neuromodulation for researchers and clinicians, our team is honoured to build world-class technology to study and treat the brain. This certification allows expanded reach to support this life-saving work.”
The European Union Medical Device Regulation (EU MDR) governs the safety, performance, and quality of medical devices sold in the European Economic Area (EEA). It replaced the previous Medical Device Directive (MDD). The certification is issued by a Notified Body, an independent organization designated by an EU member state.
“Our customers can be confident that with this certification, Magstim technology has passed some of the most rigorous quality testing medical devices are subjected to,” said Stolec-Campo. “The big thank you goes to our internal Welcony teams in Regulatory, R&D and support for your commitment to get this across the line—we know this will help people worldwide.”
As the only integrated TMS technology, Magstim is now the only TMS system which can treat MDD, Anxious Depression, OCD, and Adolescent Depression with a single treatment coil. The FDA recently cleared the Magstim Horizon 3.0 and Inspire Transcranial Magnetic Stimulation Systems for treating adolescent patients aged 15-21 for MDD, providing a non-invasive, non-pharmacological technology to improve care.
To learn more about the entire family of research and clinical neurotechnology innovations, visit Magstim.com or call 844-624-7846.
About Welcony
Globally, Welcony technologies have supported thousands of research labs, clinics, hospitals and universities that focus on mental health, brain disorders, cognitive neuroscience and neuromonitoring. Key brands include Magstim Magnetic Stimulation, MagstimEGI high-density EEG, Technomed Clinical Neurophysiology and Neurosign Interoperative Nerve Monitoring. Welcony is backed by Telegraph Hill Partners, a San Francisco based private equity company.
Media Contact: Mark Sejvar, msejvar@welcony.com, 323-363-3530
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/6739d937-ac3a-4dc8-b8a8-f6e50bf48800
- 搜索
-
- 05-20投流新工具颠覆运营经验?这些卖家已火速转向
- 05-20农发行天心区支行投放1.44亿元信贷资金支持粮棉油产业发展
- 05-20【银发族的快乐新主张】Partyhouse派对屋:让爸妈的退休生活秒变“黄金档”!
- 05-20雷平阳走进“彩云英才荟”讲述 “云南故事“助力云南文旅产业发展
- 05-20一文读懂低代码无代码平台:五大主流产品剖析
- 05-20何氏眼科完成首例新微创全飞秒SMILE pro手术!近视手术创新技术合作发布会成功举办
- 05-20熊大爷三款新面上市,开启舌尖美味新体验
- 05-20宋章萌:光影逐梦人,内外皆璀璨
- 05-20茉莉奶白五月全国百店同开,扩张势能再释放
- 05-20贵州石阡农民企业家卓仕平 向巡视组反映问题遇危险